It’s tricky to get a good look at DNA when it won’t stick to your slide, or lay down in a straight strand.
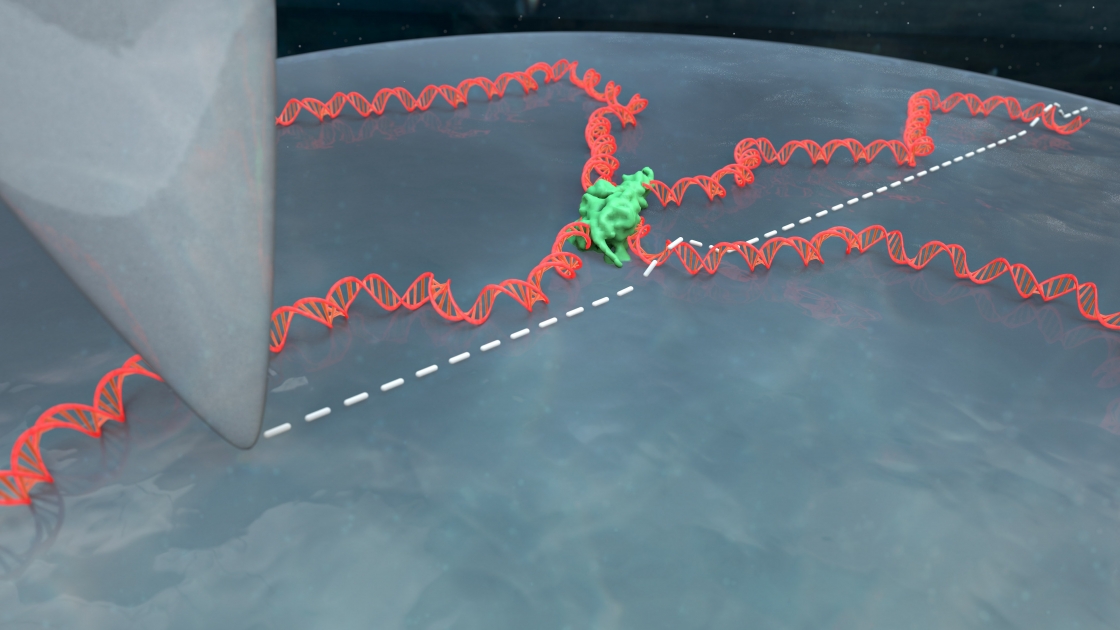
CU biochemist Tom Cech came to Tom Perkins’ atomic force microscopy lab with this very problem. His group was trying to understand how certain proteins interact with DNA. They knew their proteins interacted with DNA but they couldn’t get a good image of that interaction in liquid, DNA’s native environment.
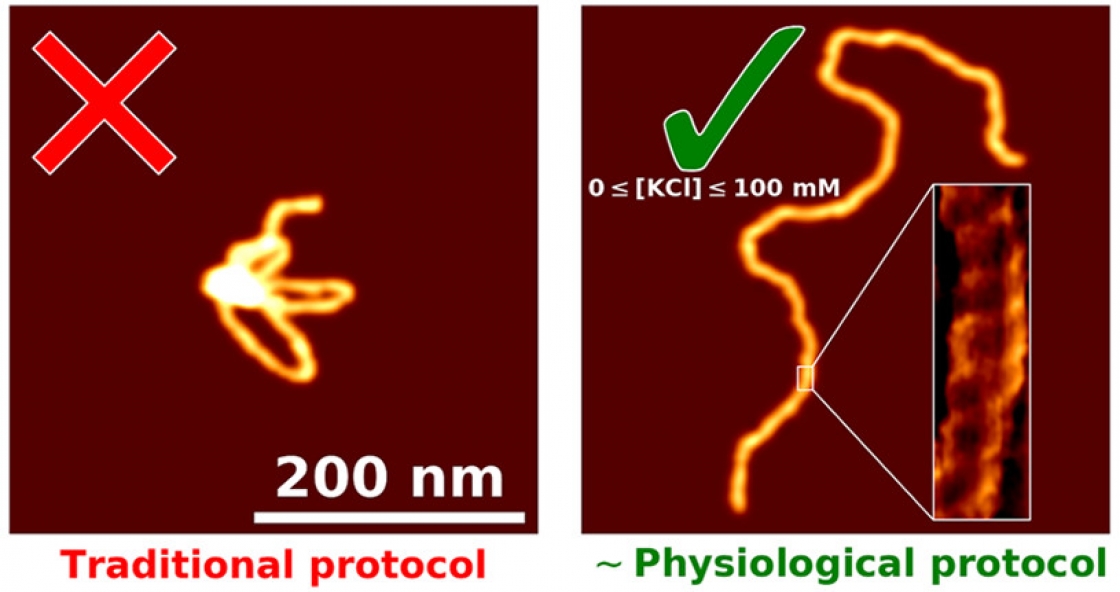
“You have this nanoscale string in a ball shape that's undergoing thermal fluctuations, and then take this three-dimensional configuration, and squish it down really fast onto a surface,” Perkins explained. That squished DNA is often an uninterpretable blob.
“I kind of think of it like a bowl of spaghetti noodles. If you drop them on the floor, they'd be kind of all globbed up and in weird shapes,” explained Patrick Heenan, a graduate student in Perkins’ group.
The goal was to develop a process that would separate those globs into individual noodles. Heenan started working on this problem about two years ago, and he developed a technique to get your slide perfectly prepared to image a DNA sample. The best part? It’s fast – ready in just five minutes.
Tiny globs of DNA
DNA is about 2 nanometers wide – 100,000 times thinner than a sheet of paper. To get a good look at it, you need to put it on a slide that is truly flat. Mica, a light, soft silicate mineral, is a great candidate for a slide. All it takes to get a flat, thin sheet of mica is a piece of Scotch tape and some deft hands, Heenan explained.
“The beauty is it's super, super flat, so it is the perfect substrate,” Heenan said. “You can see all the atoms, and in some cases, you can see voids in the lattice.”
However, mica and DNA are both negatively charged; they repel each other. Scientists had tried changing the surface of the mica to make the DNA stick – soaking it for hours or days in various coatings and rinsing it with distilled water.
But the DNA still ends up in globs. When the apex of the atomic force microscope (AFM) tip runs over the DNA, the picture comes out blurry. Scientists can’t see how proteins or other molecules are interacting with the DNA. Many had given up on imaging DNA in liquid on bare mica, figuring it didn’t work or using coatings that made the images blurrier but still yielded globs. So Heenan started digging through the literature to see what others had tried.
“I read lots of papers, and I prepped hundreds of samples,” Heenan said. “It was a little bit like whacking through the weeds.”


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.